What is a vaginal hysterectomy?
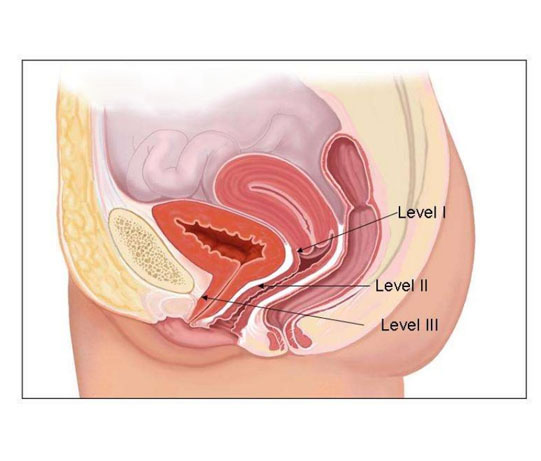
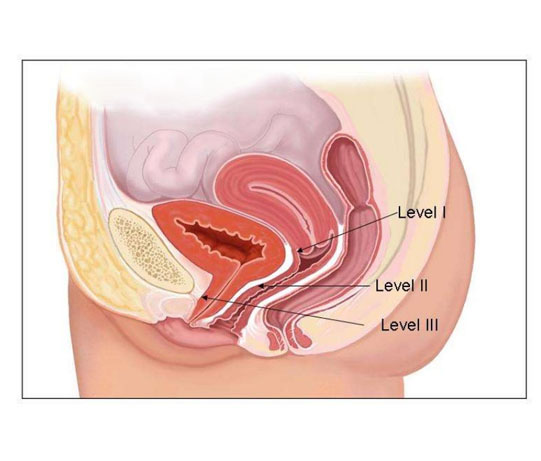
A vaginal hysterectomy is an operation to remove your uterus (womb) and cervix (neck of your womb) through your vagina. It is possible also to remove your ovaries but they will usually be left alone.
What are the benefits of surgery?

There are common reasons for having a hysterectomy.
- Heavy or painful periods.
- Fibroids, where part of the muscle of your womb becomes overgrown.
- Uterine prolapse, where your womb drops down.
A hysterectomy may cure or improve your symptoms. You will no longer have periods.
Are there any alternatives to a vaginal hysterectomy?
- Symptoms may be improved by doing pelvic floor exercises.
- Heavy periods can be treated using a variety of non-hormonal and hormonal oral (by mouth) medications. Other alternatives include an IUS (intra-uterine system - an implant containing a synthetic form of the hormone progesterone that fits in your womb) or ‘conservative surgery’ to remove the lining of your womb or prevent it from growing back.
- Depending on the size and position of fibroids, you can take medication to try to control the symptoms. Other treatments include surgery to remove the fibroids only (myomectomy) or to shrink the fibroids by reducing their blood supply (uterine artery embolisation).
What will happen if I decide not to have the operation or the operation is delayed?
Your doctor will monitor your condition and try to control your symptoms.
You may feel that you would prefer to put up with your symptoms rather than have an operation. Your gynaecologist will tell you the risks of not having an operation.
If you experience any of the following symptoms, contact your healthcare team.
- Changes to your monthly bleeding pattern if you have periods.
- Increased abdominal (tummy) swelling.
- Worsening pain that needs more medication than you are currently taking.
What does the operation involve?
The operation is usually performed under a general anaesthetic but various anaesthetic techniques are possible. The operation usually takes about 45 minutes.
Your gynaecologist will examine your vagina. They will make a cut around your cervix at the top of your vagina so they can remove your womb and cervix.
They will usually stitch the support ligaments of your womb to the top of your vagina to reduce the risk of a future prolapse and may place a pack (like a large tampon) in your vagina.
How can I prepare myself for the operation?
If you smoke, stopping smoking now may reduce your risk of developing complications and will improve your long-term health.
Try to maintain a healthy weight. You have a higher risk of developing complications if you are overweight.
Regular exercise should help to prepare you for the operation, help you to recover and improve your long-term health. Before you start exercising, ask the healthcare team or your GP for advice.
If you have not had the coronavirus (COVID-19) vaccine, you may be at an increased risk of serious illness related to COVID-19 while you recover. Speak to your doctor or healthcare team if you would like to have the vaccine.
What complications can happen?
Some complications can be serious and can even cause death.
General complications of any operation
- feeling or being sick
- bleeding
- blood clot in your leg
- blood clot in your lung
- infection of the surgical site (wound)
- allergic reaction to the equipment, materials or medication
- acute kidney injury
- chest infection
Specific complications of this operation
- pelvic infection or abscess
- developing an abnormal connection (fistula) between your bowel, bladder or ureters and your vagina
- damage to structures close to your womb
- conversion to an abdominal hysterectomy
- developing a collection of blood (haematoma) inside your abdomen
- vaginal cuff dehiscence
- recurrent prolapse
- new prolapse
Long-term problems
- developing a prolapse
- difficulty or pain having sex
- tissues can join together in an abnormal way
- passing urine more often, having uncontrolled urges to pass urine or urine leaking from your bladder when you exercise, laugh, cough or sneeze
- feelings of loss as a hysterectomy will make you infertile
- going through menopause
Consequences of this procedure
- pain
How soon will I recover?
You will be able to go home when your gynaecologist decides you are medically fit enough, which is usually after 1 to 3 days.
Rest for 2 weeks and continue to do the exercises that you were shown in hospital.
You can return to work once your doctor has said you are well enough to do so (usually after 4 to 6 weeks, depending on your type of work). You should be feeling more or less back to normal after 2 to 3 months.
Regular exercise should help you to return to normal activities as soon as possible. Before you start exercising, ask the healthcare team or your GP for advice.
Most women make a good recovery and return to normal activities.
Summary
A hysterectomy is a major operation usually recommended after simpler treatments have failed. Your symptoms should improve.
IMPORTANT INFORMATIONThe operation and treatment information on this page is published under license by Healthdirect Australia from EIDO Healthcare Australia and is protected by copyright laws. Other than for your personal, non-commercial use, you may not copy, print out, download or otherwise reproduce any of the information. The information should not replace advice that your relevant health professional would give you. Medical Illustration Copyright © Medical-Artist.com.
For more on how this information was prepared, click here.
Learn more here about the development and quality assurance of healthdirect content.
Last reviewed: September 2022